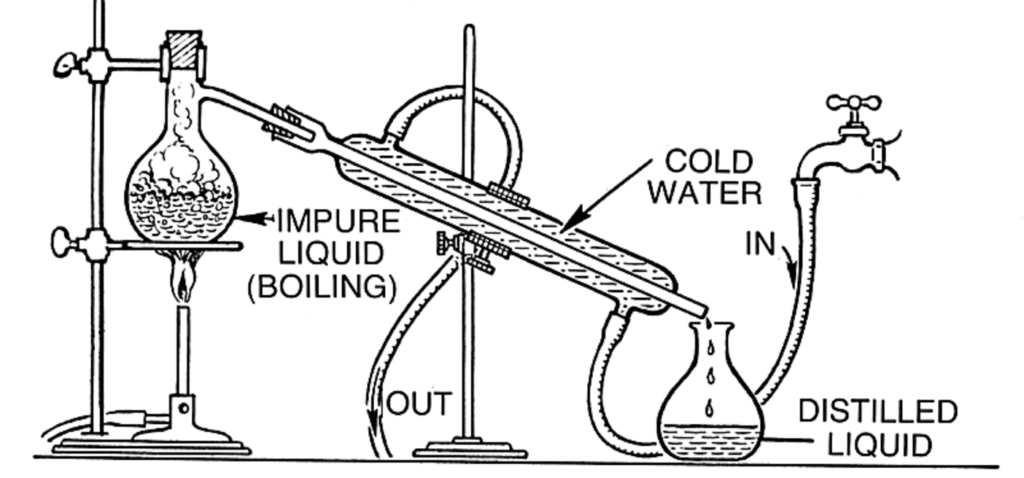
Distillation of water is a process used to purify water by separating impurities based on differences in boiling points. It involves heating water to its boiling point, collecting the steam, and then cooling the steam to condense it back into liquid form, leaving contaminants behind.
Here’s a more detailed explanation of the distillation process:
Steps in Water Distillation:
- Heating the Water:
- Water is heated in a distillation apparatus (such as a distillation flask or kettle). As the temperature rises, the water reaches its boiling point (100°C or 212°F at sea level), causing it to turn into steam. Impurities, such as salts, heavy metals, minerals, and microorganisms, remain in the liquid phase and do not evaporate.
- Evaporation:
- The water molecules, once they turn into steam, escape into the air. This purified steam is free from most contaminants, since most substances in the water (such as salts or bacteria) have higher boiling points than water and are left behind.
- Condensation:
- The steam is then directed into a cooling chamber or condenser where it cools down. As the steam cools, it condenses back into liquid water. The condenser usually has cold water running through it to help lower the temperature of the steam efficiently.
- Collection of Distilled Water:
- The condensed water is collected as purified water in a separate container. This liquid is called distilled water.
Purity of Distilled Water:
Distilled water is very pure because the distillation process removes:
- Dissolved salts
- Heavy metals (such as lead or mercury)
- Bacteria and viruses (they don’t evaporate with the water)
- Minerals (like calcium or magnesium)
- Other dissolved organic compounds
However, some volatile substances with lower boiling points than water, such as alcohols or certain chemicals, may also evaporate during distillation. These can sometimes remain in the distilled water if present in the source water.
Uses of Distilled Water:
- Laboratories and Medical Fields:
- For experiments, cleaning equipment, or producing medications where high purity is essential.
- Industrial Applications:
- Used in industries where water purity is crucial, such as in boilers, cooling systems, and in electronics manufacturing.
- Household and Personal Care:
- It’s sometimes used in car batteries, steam irons, or humidifiers to avoid mineral buildup.
- It’s used for preparing baby formula and in other food applications to ensure no impurities or contaminants affect the product.
- Drinking Water:
- While distilled water is safe to drink, it’s generally not preferred because it lacks minerals, which some believe are beneficial for health. In some cases, people may find distilled water flat or bland tasting.
Advantages of Water Distillation:
- Effective purification: It removes a wide range of contaminants, including microbes, chemicals, and heavy metals.
- Simple process: Distillation doesn’t require chemicals, just heat, making it a natural method of purification.
- High-quality water: The final distilled product is generally free from most contaminants.
Disadvantages of Water Distillation:
- Energy Intensive: Distillation requires a lot of energy to heat the water to its boiling point, which can make it more expensive and less efficient compared to other water purification methods (like filtration).
- Slow Process: The process takes time, which can be a drawback for large-scale water treatment.
- Lack of Minerals: Distilled water is demineralized, which can make it less palatable for some people and lacking in beneficial minerals like calcium and magnesium.
Types of Distillation:
- Simple Distillation: Used for purifying liquids with a significant difference in boiling points (e.g., distilling water from a mixture).
- Fractional Distillation: More complex and used to separate a mixture into several parts based on differing boiling points, such as separating various liquids in a solution.
- Vacuum Distillation: Reduces the boiling point of water by lowering the pressure, which is useful when distilling heat-sensitive substances.
- Steam Distillation: Often used for extracting essential oils, where steam is passed through plant material to separate volatile compounds.
Distillation vs. Other Water Purification Methods:
- Filtration: Filters can remove large particles, but they may not eliminate dissolved chemicals, minerals, or microorganisms. Distillation provides a higher level of purity.
- Reverse Osmosis: Uses a semipermeable membrane to remove impurities from water. It’s highly effective, but it uses energy and can waste some water.
- Boiling: Kills bacteria and viruses but does not remove chemical contaminants or minerals.
In summary, distillation is a highly effective way to purify water by separating water from its contaminants based on differences in boiling points. While energy-intensive and slow, it provides very pure water, making it ideal for certain applications where high water quality is crucial.

Dinesh gangwar
Excellent performance by students